Magnesium (Mg) plays an important role in controlling bone apatite structure and density and is a potential bioactive material in repairing critical-sized bone defects. In this study, we aimed to evaluate the effect of adding NanoMgO to polycaprolactone/beta-tricalcium phosphate (PCL/β-TCP) scaffolds on bone regeneration.
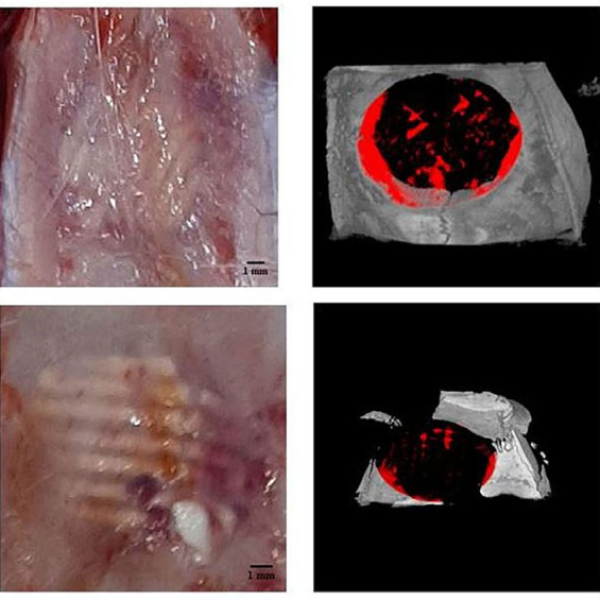
Novel 3D-printed porous PCL/β-TCP composite scaffolds containing 10% nanoMgO were fabricated by fused deposition modeling (FDM) and compared with PCL/β-TCP (1:1) scaffolds (control). The morphology and physicochemical properties of the scaffolds were characterized by ATR-FTIR, XRD, scanning electron microscope-energy dispersive X-ray analysis (SEM–EDX), transmission-electron-microscopy (TEM), water contact angle, and compressive strength tests and correlated to its cytocompatibility and osteogenic capacity in-vitro. To evaluate in-vivo osteogenic capacity, bone-marrow-derived stem cell (BMSC)-loaded scaffolds were implanted into 8 mm rat critical-sized calvarial defects for 12 weeks.
The hydrophilic scaffolds showed 50% porosity (pore size = 504 μm). MgO nanoparticles (91.5 ± 27.6 nm) were homogenously dispersed and did not adversely affect BMSCs' viability and differentiation. Magnesium significantly increased elastic modulus, pH, and degradation. New bone formation (NBF) in Micro-CT was 30.16 ± 0.31% and 23.56 ± 1.76% in PCL/β-TCP/nanoMgO scaffolds with and without BMSCs respectively, and 19.38 ± 2.15% and 15.75 ± 2.24% in PCL/β-TCP scaffolds with and without BMSCs respectively. Angiogenesis was least remarkable in PCL/β-TCP compared with other groups (p < .05).
Our results suggest that the PCL/β-TCP/nanoMgO scaffold is a more suitable bone substitute compared to PCL/β-TCP in critical-sized calvarial defects.