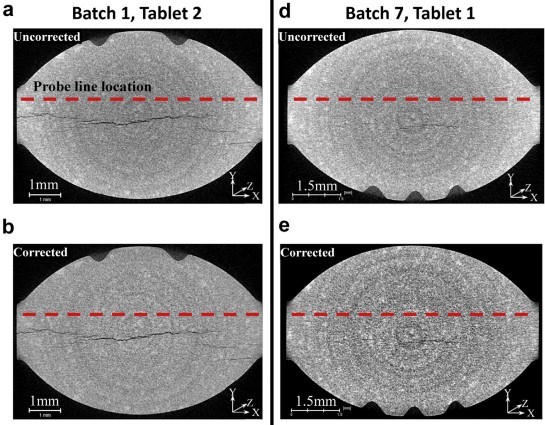
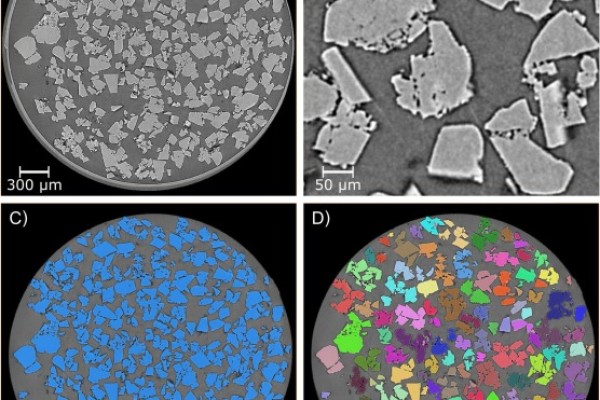
Physical tablet defects are related to internal structural defects that are not easily assessed by the traditional methods, such as dusting, laminating, or fracturing during appearance, friability, or hardness testing. Also, these methods do not allow objective and quantitative investigation of the role of formulation and process variables, which is essential for quality-by-design drug product development. In this study, an X-ray microcomputed tomography (XμCT) method to analyze internal tablet defects is developed using tablets from a quality-by-design design-of-experiment study. The design of experiment investigated the effect of roller compaction roll force, filler composition, and the amount of magnesium stearate on tablet quality attributes. Average contiguous void volume by optical image processing and fracture size distribution and direction by artificial intelligence-based image processing quantified the internal tablet fracture severity. XμCT increased formulation and process knowledge in support of scale-up manufacturing. We demonstrated how XμCT can be incorporated as a part of a holistic approach to quantitatively identify and mechanistically assess the risks of internal tablet defects. Furthermore, expanding the use of XμCT with an artificial intelligence-based quantitative analysis can deepen our tableting knowledge from an empirical understanding to a mechanistic understanding of compaction phenomenon.